Towards a Machine-Learning-Based Application for Amorphous Drug Recognition
Keywords:
drug amorphization, monte-carlo method, deep neural networkAbstract
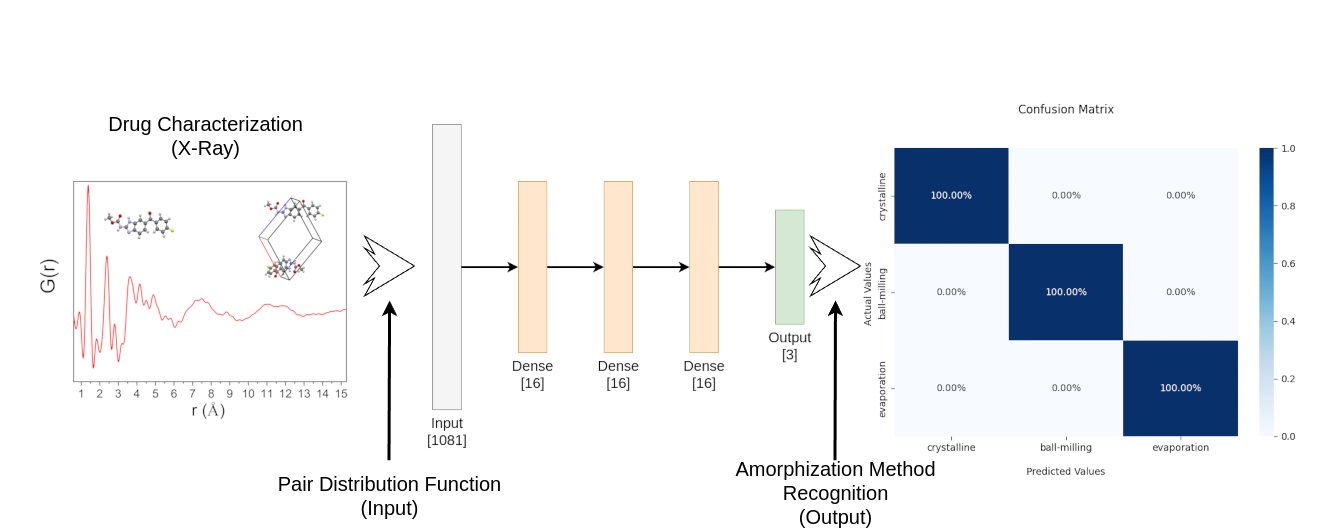
The amorphous drug structure represents an important feature to be reached in the pharmaceutical field due to its possibility of increasing drug solubility, considering that at least 40% of commercially available crystalline drugs are poorly soluble in water. However, it is known that the amorphous local structure can vary depending on the amorphization technique used. Therefore, recognizing such variations related to a specific amorphization technique through the pair distribution function (PDF) method, for example, is an important tool for drug characterization concerns. This work presents a method to classify amorphous drugs according to their amorphization techniques and related to the local structure variations using machine learning. We used experimental PDF patterns obtained from low-energy X-rays scattering data to extract information and expanded the data through the Monte Carlo method to create a synthetic dataset. Then, we proposed the evaluation of such a technique using a Deep Neural Network. Based on the results obtained, it is suggested that the proposed technique is suitable for the amorphization technique and local structure recognition task.
Downloads
References
P. H. Karpinski, “Polymorphism of active pharmaceutical ingredients,”
Chemical Engineering & Technology, vol. 29, no. 2, pp. 233–237,
doi: https://doi.org/10.1002/ceat.200500397. [Online]. Available:
https://onlinelibrary.wiley.com/doi/abs/10.1002/ceat.200500397
S. Bates, R. C. Kelly, I. Ivanisevic, P. Schields, G. Zografi,
and A. W. Newman, “Assessment of defects and amorphous
structure produced in raffinose pentahydrate upon dehydration,”
Journal of Pharmaceutical Sciences, vol. 96, no. 5, pp. 1418–
, 2007. doi: https://doi.org/10.1002/jps.20944. [Online]. Available:
https://www.sciencedirect.com/science/article/pii/S0022354916322523
L. Yu, “Amorphous pharmaceutical solids: preparation, characterization
and stabilization,” Advanced Drug Delivery Reviews, vol. 48, no. 1,
pp. 27–42, 2001. doi: https://doi.org/10.1016/S0169-409X(01)00098-
Characterization of the Solid State. [Online]. Available: https:
//www.sciencedirect.com/science/article/pii/S0169409X01000989
S. J. L. Billinge, T. Dykhne, P. Juhás, E. Božin, R. Taylor, A. J. Florence,
and K. Shankland, “Characterisation of amorphous and nanocrystalline
molecular materials by total scattering,” CrystEngComm, vol. 12,
pp. 1366–1368, 2010. doi: 10.1039/B915453A. [Online]. Available:
http://dx.doi.org/10.1039/B915453A
I. C. B. Martins, A. S. Larsen, A. Ø. Madsen, O. A. Frederiksen,
A. Correia, K. M. Ø. Jensen, H. S. Jeppesen, and T. Rades, “Unveiling
polyamorphism and polyamorphic interconversions in pharmaceuticals:
the peculiar case of hydrochlorothiazide,” Chem. Sci., vol. 14, pp.
447–11 455, 2023. doi: 10.1039/D3SC02802J. [Online]. Available:
http://dx.doi.org/10.1039/D3SC02802J
J. Jiang, D. Ouyang, and R. O. Williams III, “Predicting glass-forming
ability of pharmaceutical compounds by using machine learning
technologies,” AAPS PharmSciTech, vol. 24, no. 5, p. 103, 2023.
[Online]. Available: https://doi.org/10.1208/s12249-023-02535-610.
/s12249-023-02535-6
J. Jiang, A. Lu, X. Ma, D. Ouyang, and R. O. Williams III, “The
applications of machine learning to predict the forming of chemically
stable amorphous solid dispersions prepared by hot-melt extrusion,”
International Journal of Pharmaceutics: X, vol. 5, p. 100164, 2023.
doi: https://doi.org/10.1016/j.ijpx.2023.100164. [Online]. Available:
https://www.sciencedirect.com/science/article/pii/S2590156723000087
A. Alhalaweh, A. Alzghoul, W. Kaialy, D. Mahlin, and C. A. Bergström,
“Computational predictions of glass-forming ability and crystallization
tendency of drug molecules,” Molecular pharmaceutics, vol. 11,
no. 9, pp. 3123–3132, 2014. doi: https://dx.doi.org/10.1021/mp500303a.
[Online]. Available: https://pubs.acs.org/doi/full/10.1021/mp500303a
E. Fink, M. Brunsteiner, S. Mitsche, H. Schröttner, A. Paudel,
and S. Zellnitz-Neugebauer, “Data-driven prediction of the formation
of co-amorphous systems,” Pharmaceutics, vol. 15, no. 2, p.
, 2023. doi: 10.3390/pharmaceutics15020347. [Online]. Available:
https://www.mdpi.com/1999-4923/15/2/347
H. C. Nguyen, F. Alamray, M. Kamal, T. Diana, A. Mohamed,
M. Algarni, and C.-H. Su, “Computational prediction of drug
solubility in supercritical carbon dioxide: Thermodynamic and artificial
intelligence modeling,” Journal of Molecular Liquids, vol. 354, p.
, 2022. doi: 10.1016/j.molliq.2022.118888. [Online]. Available:
https://www.sciencedirect.com/science/article/pii/S0167732222004263
T. G. Geary, C. D. Mackenzie, and S. A. Silber, “Flubendazole as
a macrofilaricide: History and background,” PLoS neglected tropical
diseases, vol. 13, no. 1, p. e0006436, 2019.
R. L. Harrison, “Introduction to monte carlo simulation,” in AIP
conference proceedings, vol. 1204, no. 1. American Institute of Physics,
doi: https://doi.org/10.1063/1.3295638 pp. 17–21. [Online].
Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2924739/
M. C. Silva, J. C. F. da Silva, and R. A. R.
Oliveira, “Idissc: Edge-computing-based intelligent diagnosis
support system for citrus inspection.” in ICEIS (1),
doi: https://doi.org/10.5220/0010444106850692 pp. 685–692.
[Online]. Available: https://www.scitepress.org/Link.aspx?doi=10.5220/